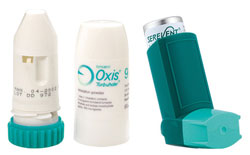
Long-acting Beta Agonists and Asthma
The safety of long-acting beta agonists (LABAs) is being questioned again after the Food and Drug Administration (FDA) banned two drugs and issued warnings about two others. An expert panel to the FDA voted on 11th December 2008 to ban the asthma drugs Serevent (Salmeterol) and Foradil, sold as Oxis in New Zealand. The FDA’s safety office are also recommending that Advair (Seretide) and Symbicort no longer be used to treat children with asthma because of the risk of serious breathing problems.
The FDA ban will come as a shock to many asthma sufferers and their doctors in Australia and New Zealand where there has been a well funded campaign to get all patients, regardless of asthma severity, onto these drugs. LABAs were originally developed for asthma that cannot be controlled with conventional inhaled corticosteroid medications and short-acting beta agonist relievers when needed. Unfortunately they are now widely prescribed with disregard to the numerous warnings.
There is now a large body of evidence going back tothe Fenoterol morbidities in the 1970s documenting the role of bronchodilators on worsening asthma. Julian Crane, Professor, Department of Medicine, Wellington School of Medicine in a letter to the New Zealand Medical Journal July 2006 warned that ‘It is unfortunate that we still do not know how some short-acting beta agonists such as isoprenaline and Fenoterol or the long-acting beta agonists (LABAs) increase the risk of death in severe asthma, but in the case of LABAs it appears to happen without excessive use.’
One theory put forward by proponents of breathing re-training is that asthma is a compensatory response to over-breathing in genetically susceptible individuals. The problem posed by short- and long-acting beta agonists is that they increase breathing through adrenal stimulation: the more bronchodilator taken, the worse the asthma. Long-acting beta agonists deliver considerably higher doses of the bronchodilator drugs so pose a higher risk of asthma exacerbations and, in some cases, death.
Asthma patients and their doctors are generally well informed about drug treatments but up until now little emphasis has been placed on physical coping skills and the importance of correct breathing. Dependency on asthma medication is regarded as the norm, and there is little encouragement for people with asthma to try alternative methods to control their breathing.
There are now more than seven published studies demonstrating the efficacy of breathing retraining programmes such as Buteyko in asthma management. The British Guideline on the Management of Asthma 2008 grants permission for British health professionals to recommend Buteyko, stating that the method may be considered to help patients control the symptoms of asthma. The guideline also grades clinical research on Buteyko with a ‘B’ classification – indicating that high-quality supporting clinical trials are available.
More research is required before Buteyko or other breathing retraining approaches are fully incorporated into general practice. However, this research needs to happen sooner rather than later if we are to curb the mortality and morbidity resulting from beta agonist use.
Glenn White BSc MSc
Practitioner member Buteyko Institute of Breathing and Health