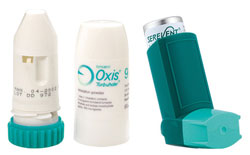
An FDA Panel said Thursday that the risks of death and serious injury associated with two popular brands of asthma inhalers are greater than the benefits of the drugs. However, in what is sure to be a controversial opinion, the panel said that Advair (Symbicort) should continue to be used to treat asthma.
The FDA has previously said that Serevent, Foradil (sold as Oxis in NZ), and similar drugs are linked to an increased risk of asthma-related side effects, particularly in children and the elderly. The side effects have resulted in injuries including asthma-related death, hospitalization, and asthma-related intubations, where a tube must be placed in patients’ noses or mouths to help them breathe.
The agency found an additional 2.8 asthma events per 1,000 patients treated with a LABA compared with patients not receiving the drugs. Note: Long-acting beta agonists (LABAS) are sold in NZ as Serevent, Oxis, and in combination with inhaled corticosteroid asthma drugs as Symbicort and Seretide.